As a mother, Brannon Traxler wanted to protect her infant daughter against COVID-19.
As the public health director for the South Carolina Department of Health and Environmental Control, Dr. Brannon Traxler was confident the vaccines were safe.
“I very much trust the process that these vaccines and really all vaccines and medications and devices go through with the FDA. So, I had full trust that if it had gotten to this point in clinical trials that it was safe,” she said.
So much so, Traxler enrolled her six-month old daughter, Lucy, in clinical trials.
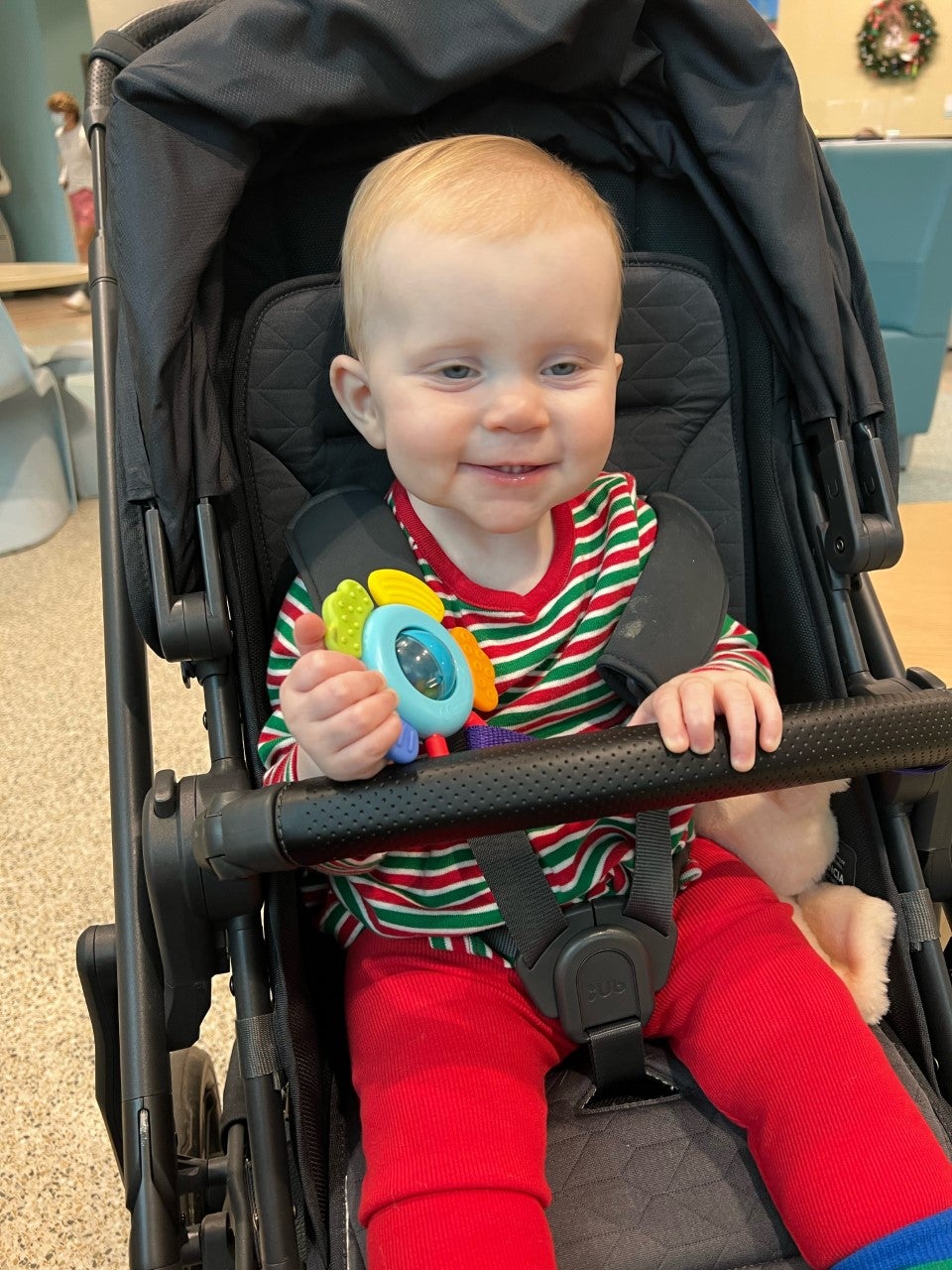
“I went about this as an average South Carolinian. This had nothing to do with my professional role,” she said. “I read an article about how MUSC (Medical University of South Carolina) was going to be participating in this trial and it included a link for where you could sign up if you’re interested in your child participating. So, I signed up.”
Signing up simply got Lucy into the pool of children enrolled in the clinical trial. It was no guarantee that she would receive a vaccine or would get a placebo instead. She received the first injection when she was nine months old.
The trial through MUSC was a double-blind trial, meaning neither the patients nor the doctors knew which study group the patients were in.
The Food and Drug Administration granted the Emergency Use Authorization on June 17 for both the Pfizer-BioNTech and Moderna vaccines to be administered to children as young as six months old.
It was that same day Traxler learned her daughter was in the group who received the Moderna vaccine.
The following day, Dr. Rochelle Walensky, director of the Centers for Disease Control and Prevention, endorsed recommending vaccinations for children aged six months through age five.
“I encourage parents and caregivers with questions to talk to their doctor, nurse, or local pharmacist to learn more about the benefits of vaccinations and the importance of protecting their children by getting them vaccinated,” said Walensky.
It is advice Traxler supports while understanding some parents are still hesitant to have their child vaccinated against SARS-CoV-2.
“I understand as a parent, and as a parent who did not become a parent easily. It was not an easy journey for us to become parents. I understand how much you love your child and how much you want to protect them. And when there are unknowns that’s scary,” she said. “I just want them to know that not only do the numbers say that it’s safe and effective, but I tell them she had no side effects whatsoever. They need to talk to a medical professional that they trust, especially one that knows their child, who can also help answer questions and give them accurate information.”
Moderna’s vaccine for individuals aged six months to 17 years of age will be administered as a primary series of two doses, one month apart. Individuals in that age group who are immunocompromised are eligible for a third primary dose a month after completing the two-dose series.
It’s a three dose primary series for the Pfizer-BioNTech vaccine. The initial two doses are administered three weeks apart. The third dose follows at least eight weeks after the second dose.

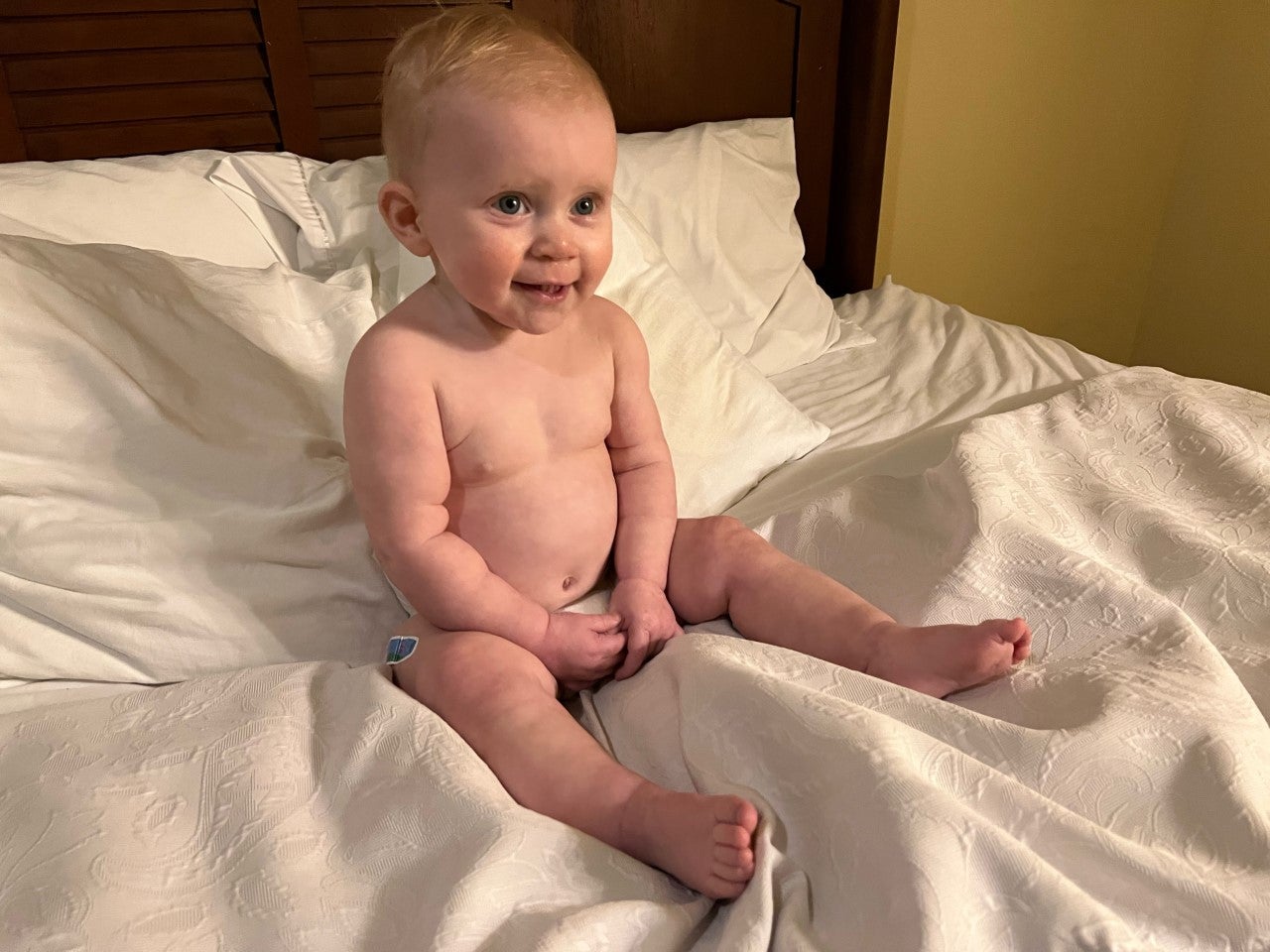
Now 17 months old, Lucy Traxler will continue to be part of the research on COVID-19. She is enrolled in a study on Long COVID in children that will continue for several years. Lucy will be part of the control group.
While the toddler is too young to understand what she has done, and the significance, MUSC gave her a little bear to mark her participation. A bear with a cape and a mask. A hero bear. Brannon Traxler has taken photos to share with Lucy as she grows up.
“I look forward to one day when she’s older and can understand. Being able to tell her that she really was a public health hero and helped to make these vaccines available for everyone,” Traxler said.
Dana Lynn McIntyre is a general assignment reporter for The Augusta Press. Reach her at dana@theaugustapress.com











