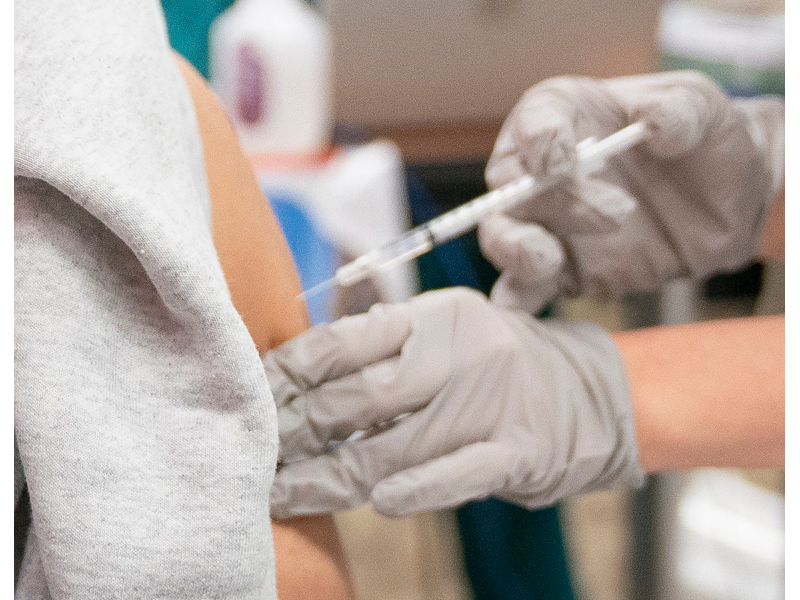Final approval is expected by Thursday allowing children aged 12 to 15 to be vaccinated with the Pfizer COVID-19 vaccine.
The FDA has issued an emergency use authorization (EAU). The Advisory Committee on Immunization Practices (ACIP), which is the advisory board to the U.S. Centers for Disease Control and Prevention (CDC), is meeting Wednesday to make a recommendation. The final announcement will come from the CDC.
Spokesman Nancy Nydam confirms the Georgia Department of Public Health began vaccinating the younger age group on Tuesday following the FDA announcing the EUA.
Locally, Augusta University has the bulk of the Pfizer vaccine and is looking at ways to vaccinate the younger children.
Dr. Phillip Coule said, “In particular, we’re most concerned about the children that have significant medical conditions, chronic conditions, that put them at risk for COVID-19.”
He said that includes children with sickle cell, cystic fibrosis, bad asthma and obesity.
He said they will begin lowering the age at their vaccination clinics but will also look at targeting specific groups.
“For example, in our sickle cell clinic, cystic fibrosis clinic, things like that, we’ll be able to get those kids vaccinated,” he explained.
The expanded age group also has state departments of health, state departments of education and local school districts looking at how to vaccinate the 12- to 15-year-olds, as well as coordinate parental involvement.
Columbia County School District spokeswoman Abbigail Remkus said, “The Public Health Departments, local pharmacies and physicians stand ready to provide vaccinations to all ages that are eligible for the COVID-19 vaccine. Once the FDA releases the age limits for the vaccines, parents are encouraged to make appointments for the vaccination.”
In South Carolina, a statement from the Department of Health and Environmental Control said parents and legal guardians must consent for a child aged 12 to 15 to be vaccinated.
The statement added, “We’ve also received notice that VAMS (Vaccine Administration Management System) is working to deploy a parental consent component into the VAMS system in the coming days, which may be able to replace the need for a paper consent form from a provider. A copy of the current EUA fact sheet for the Pfizer vaccine will also be provided at the time of consent or when they receive their first shot.”
That requirement is echoed by the South Carolina Department of Education. Spokesman Ryan Brown said, “State law allows students aged 16 and older to consent to vaccinate without parent approval but districts are encouraging parents to play a role in this process. Students aged 12-15 will require parent consent to be vaccinated.”
Mike Rozier, Director of Communications for the Aiken School District said, “We’ve had some brief discussions regarding possible student clinics for the 2021-22 school year, but nothing for the current school year.”
Dana Lynn McIntyre is a Staff Reporter with The Augusta Press. You can reach her at dana@theaugustapress.com.