Moderna announced it filed a request with the Food and Drug Administration for Emergency Use Authorization of its COVID-19 vaccine in children six months to six years of age.
The request, filed April 28, said a study showed a robust antibody response after a two-dose, primary series of shots. The reported was based on results of cases collected primarily during the Omicron surge.
“We are proud to share that we have initiated our EUA submission for authorization for our COVID-19 vaccine for young children,” said Stéphane Bancel, Chief Executive Officer of Moderna, in a statement. “We believe mRNA-1273 will be able to safely protect these children against SARS-CoV-2, which is so important in our continued fight against COVID-19 and will be especially welcomed by parents and caregivers.”
The FDA is holding June 8, 21 and 22 to schedule meetings to discuss updates for both Moderna and Pfizer-BioNTech potential use on younger populations.
“As we continue to address the ongoing COVID-19 pandemic, there are a number of anticipated submissions and scientific questions that will benefit from discussion with our advisory committee members,” said Dr. Peter Marks, director of the Center for Biologics Evaluation and Research. “We are providing a tentative schedule for discussion of these submissions, as these meetings will cover a number of topics that are of great interest to the general public.”
Moderna’s announcement came just two days after the Centers for Disease Control and Prevention held a media briefing on the current trends of SARS-CoV-2 infections.
Results on testing blood samples exclusively for antibodies that developed due to infection by the virus found 58% of those tested carried antibodies resulting from infection, not vaccination.
“This study was conducted on clinical blood samples from all parts of the country,” said Dr. Kristie E.N. Clarke, co-lead for COVID-19 Epidemiology & Surveillance Taskforce Seroprevalence Team. “By February 2022, evidence of previous COVID-19 infections substantially increased among every age group compared especially to the summer 2021. The highest jump of antibody detection was among children and adolescents overall between December 2021 and February 2022.”
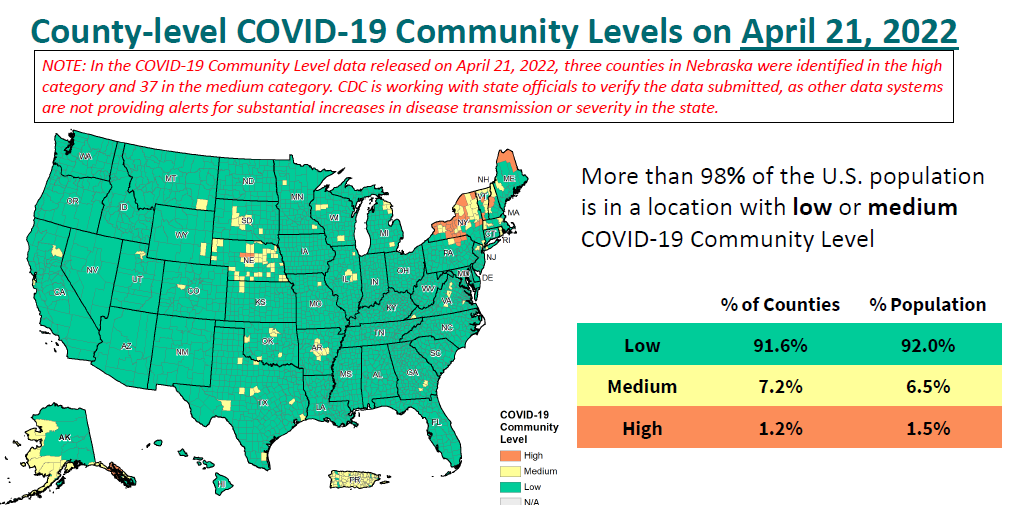
CDC Director, Dr. Rochelle Walensky, said the agency’s map illustrating community levels shows more than 98% of Americans live in an area of low or medium virus levels. One except is the Northeast which is seeing some high levels.
“We’re sequencing 1000s of viruses per week through a national SARS-CoV-2 strain surveillance and contracts with diagnostic and sequencing groups across the country to understand what is out there in the United States. Essentially 100% of what we’re finding now is Omicron. Those different lineages or sub lineages may be more common in each region. This means that if a new variant were starting to spread, we would identify it quickly,” she said.
Walensky said they were disappointed by the recent court ruling that ended mandatory mask wearing during travel. She said the CDC continues to recommend people wear masks in all indoor, public transportation settings like buses, trains and airplanes.
Dana Lynn McIntyre is a general assignment reporter for The Augusta Press. Reach her at dana@theaugustapress.com