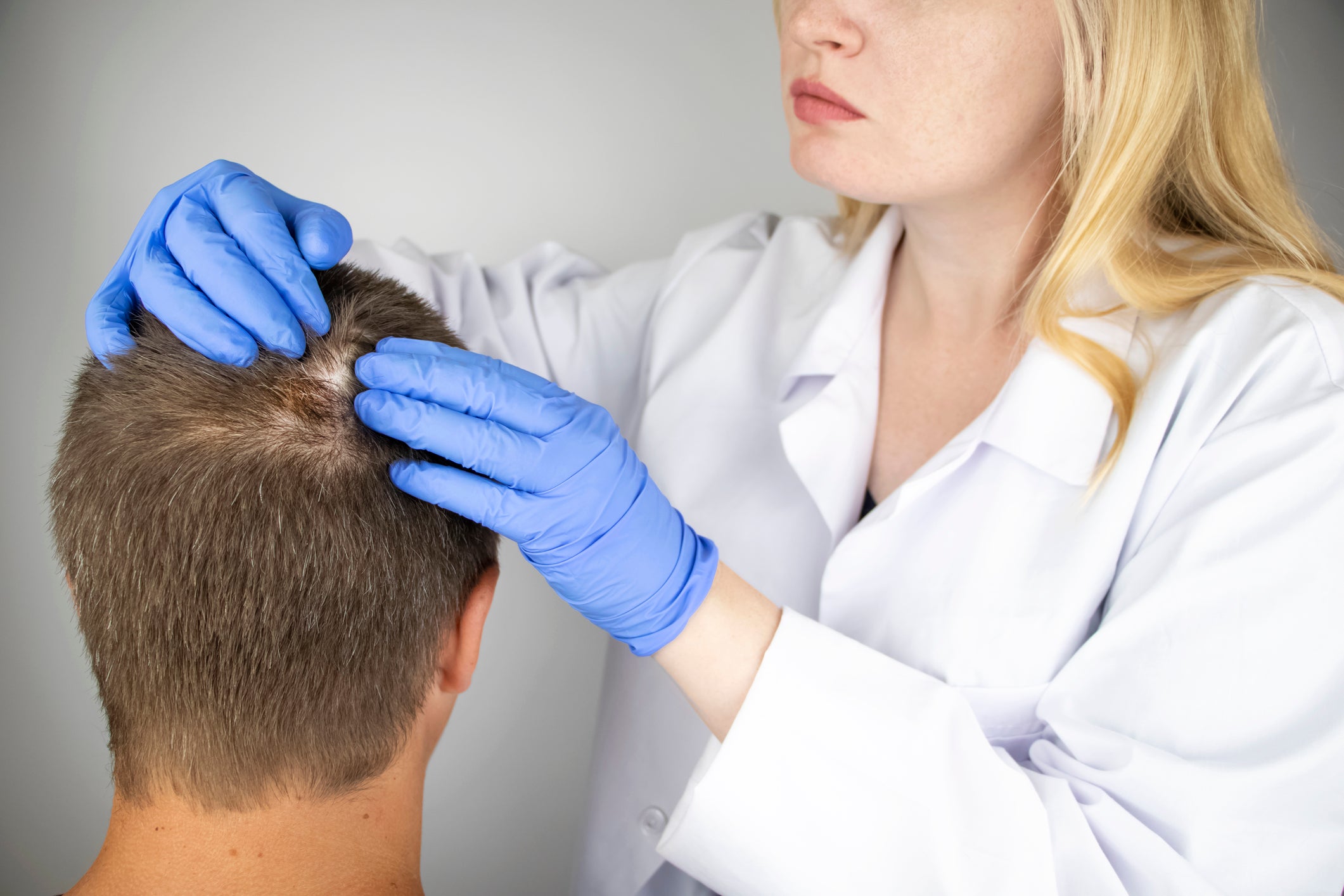
The Food and Drug Administration has approved an oral medication for patients suffering from one, specific type of hair loss.
Alopecia areata is an autoimmune disease which causes the body to attack its hair follicles. The National Alopecia Areata Foundation says there are three types of the disease:
“Alopecia areata patchy, the most common form, with one or more coin-sized hairless patches on the scalp or other areas of the body; Alopecia totalis, total loss of the hair on the scalp; Alopecia universalis, complete loss of hair on the scalp, face and body.”
MORE: Healthcare professionals keeping an eye on rising COVID-19 cases and hospitalizations
On June 13, the FDA approved the use of the drug Olumiant to treat patients with severe alopecia areata.
“Today’s approval will help fulfill a significant unmet need for patients with severe alopecia areata,” said Dr. Kendall Marcus, director of the Division of Dermatology and Dentistry in the FDA’s Center for Drug Evaluation and Research.
Dr. Kathryn Potter, a dermatologist at Augusta University Health, cautioned the new drug is only for patients who suffer from alopecia areata. She said approval of Olumiant is exciting for those patients.
“Up until now we’ve been treating with off label, you know, steroid injections and topical steroids. This will give us an actual FDA approved treatment for alopecia areata, which is nice for several reasons. It should help with insurance coverage and just give patients who haven’t had any other options up until now,” she said.
Alopecia areata is one of three types of non-scarring forms of the disease. Another is hormonal hair loss, commonly seen as male and female pattern hair loss that happens as people age.
The third is telogen effluvium, which is hair loss about three months after a major life event.
“For example, a big surgery or having a baby. But we’ve seen it a lot in the last couple of years because the stress of COVID just in general or having COVID. We’ve seen a lot of this telogen,” Potter said, adding doctors often recommend using Rogaine, which keeps hair in the growing stage longer.
Alopecia areata usually presents as small, round patches as the patient’s immune cells attack their hair follicles. For some people it can progress to alopecia universalis.
MORE: VA exoskeleton helps paralyzed vets walk again
“That is loss of all the hair, including eyebrows, eyelashes and body hair as well. So that’s much more rare. But it can happen and up until now we really didn’t have any great treatments for that,” said Potter.
“Access to safe and effective treatment options is crucial for the significant number of Americans affected by severe alopecia,” said Marcus.
Potter said even the new drug may not be appropriate for all alopecia areata patients. Olumiant is a Janus kinase (JAK) inhibitor. It blocks the activity of a specific family of enzymes, interfering with the pathway that leads to inflammation. It had been previously approved for treatment of rheumatoid arthritis and can increase the risk of clotting and blood abnormalities.
The FDA said most common side effects associated with Olumiant include upper and lower respiratory tract infections, headache, acne, high cholesterol, urinary tract infection, fatigue, nausea and weight gain.
Dana Lynn McIntyre is a general assignment reporter for The Augusta Press. Reach her at dana@theaugustapress.com